Tony Goovaerts
RN, Nurse Manager of Pre-Dialysis Education Programme, Peritoneal Dialysis and Home HD programme
Cliniques Universitaires St. Luc, Brussels, Belgium
tony.goovaerts@telenet.be
5.0 Vascular Access for Home Haemodialysis
Learning Outcomes
- To understand how different vascular access types can be used safely for HHD
- To understand how cannulation techniques can be taught to the patient/carer
- To identify possible complications and know how to limit these
Introduction
To perform safe and manageable home dialysis, a well-functioning access is vital. Access problems can cause undesirable stress in the home situation and, as a last resort, a transfer back to the hospital might be necessary, thus compromising the quality of life and independence of the patient1.
There are three types of vascular access, Arterio-Venous Fistula (AVF), Arterio-Venous Graft (AVG), Central Venous Catheter (CVC), and all can be used in the home setting.
5.1 General Information
Many HHD patients dialyse more intensively which may be associated with increased risk of vascular access adverse events2,12. Therefore, educating patients/carers in the care and management of their access, is a very important part of the training programme. At each outpatient clinic or home visit, the vascular access should be inspected. Access flow should be measured during training as a reference point for further follow-up in the home setting.
Ideally the training should start with a permanent vascular access. If this is not possible, training can be done with a CVC and the patient sent home at the end of training. When the permanent access is ready to be used, retraining for cannulation can be scheduled.
Patients on dialysis are prone to have more Staphylococcus Aureus on their skin and in their nose than the general population3,4. Therefore, whatever vascular access they may have, patients may need to be screened, and treated depending on unit protocol. Handwashing must be done perfectly.
The use of gloves for the patient in the home setting is debatable.
Gloves may inhibit the patient’s ability to maintain aseptic technique (than with bare hands thoroughly disinfected with an alcohol-based rub). Unit policies vary.
Retraining and/or home visit to observe technique is recommended, certainly after an infection episode.
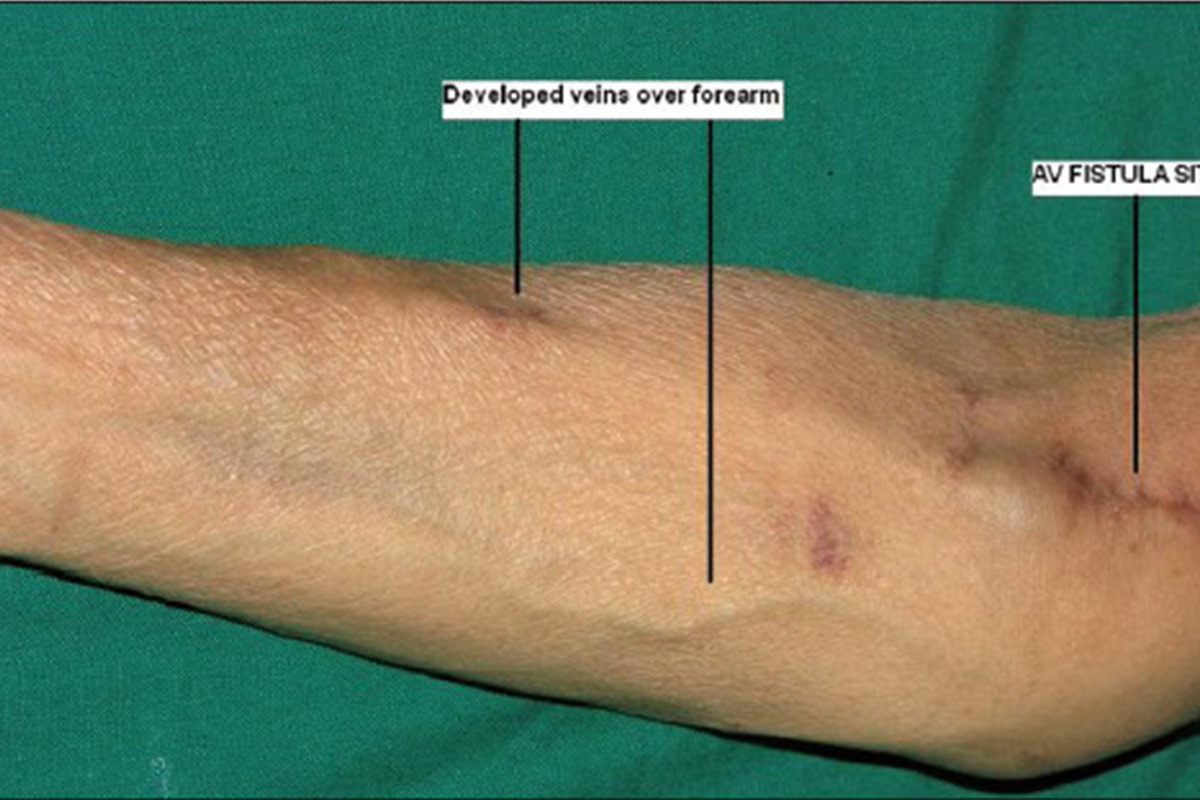
5.2 Arterio-Venous Fistula (AVF)
Although frequent HD is associated with an increased risk of vascular access complications, reported event rates were lower in the AVF group12. In-centre patients who are on a waiting list for HHD can be trained already to self-cannulate. This might shorten the actual total training time.
The ideal place for an AVF in a HHD patient who self-cannulates, is the non-dominant forearm. Upper arm AVF and AVF on the dominant arm, are more difficult to self-cannulate but it can be learned.
For more general information on AVF, see EDTNA/ERCA publication on ‘Vascular Access Cannulation and Care – a Nursing Best Practice Guide for Arteriovenous Fistula48.
“My nurse said that once I had learnt to needle myself, I would never let anyone else do it, and he was absolutely right!” – Stuart
5.2.1 Importance of self-cannulation
- Self-cannulation is a major step towards enabling self-care at home and remains the foremost barrier for its uptake5.
- By self-cannulating, patients will feel empowered in their own care, and the benefit of a single cannulator will increase the life of the vascular access6. Patients will become experts of their own access.
- In most HHD programmes self-cannulation is promoted, even if the patient has a carer. Indeed, cannulation by the carer can lead to very stressful situations. It should be limited to cases where the patient is not able to self-cannulate i.e. tremor, vision problems, inability to reach puncture sites, needle phobia.
“Self-needling is like the difference between being a passenger and the driver of a car on a twisting mountain road. The passenger may feel uncomfortable or sick. As the driver, though, you are in control and you feel fine.”
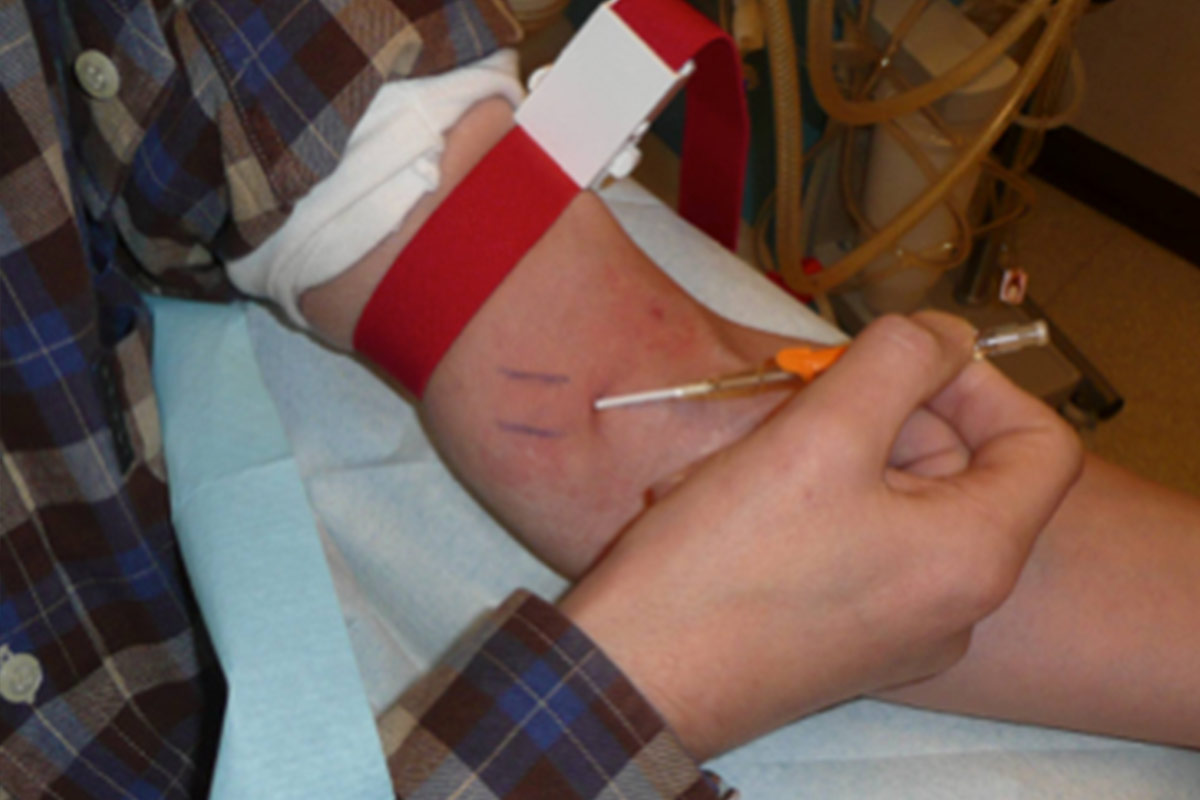
5.2.2 Practical instructions for self-cannulation
Prepare
- Cannulate in a clean and draught-free environment with a good light source.
- Proper hand washing (with liquid soap) is mandatory before starting to prepare cannulation equipment.
- Materials required: disinfectant, drape, sterile dressing pack, tape, syringes, needles, tourniquet, waste bin, sharps container.
- Disinfect hands with an alcohol based rub before aseptic preparation of syringes, prefilling of needles and tape.
- Washing (scrubbing) arm before disinfection is extremely important.
- Whatever skin disinfectant agent is used, the contact time should be respected.
- Wearing of a mask is advised but unit policies do vary.
- Washing/scrubbing of arm and washing the access arm with liquid soap and water is mandatory in order to remove as many skin bacteria as possible.
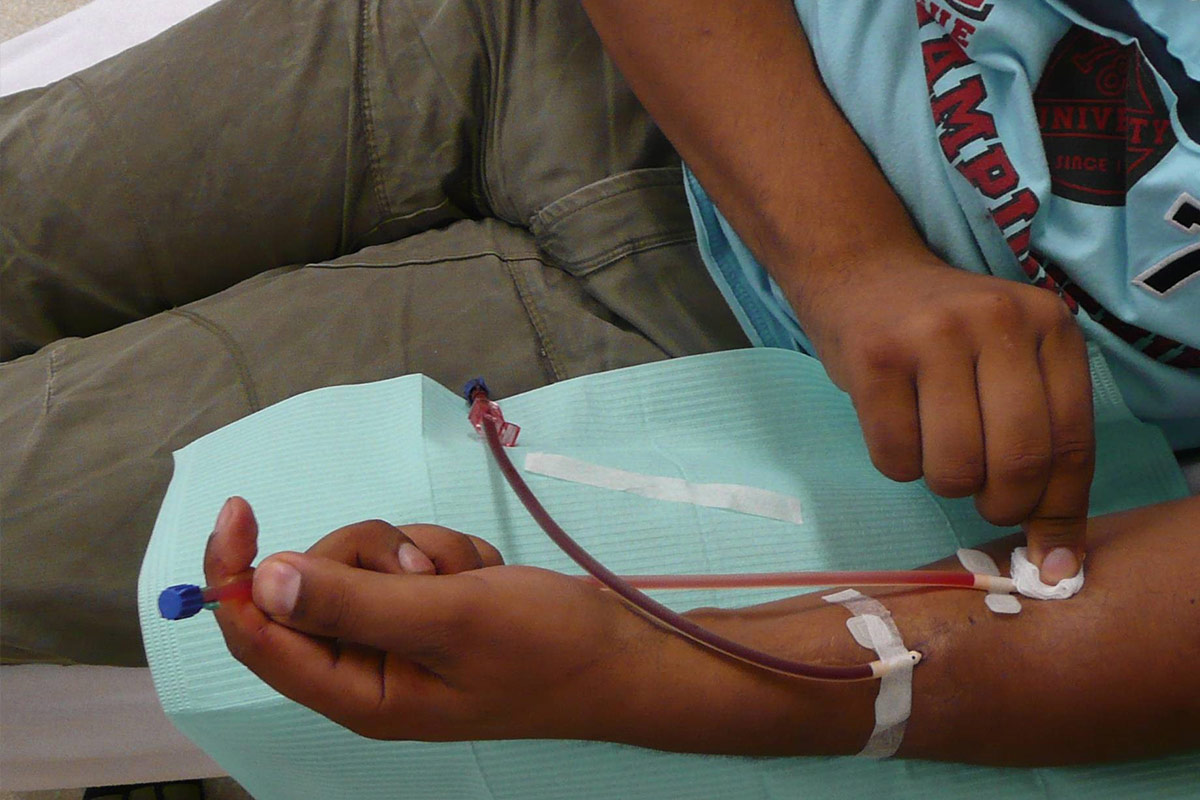
Cannulate (standard needle)
- Assessment of access must be done at each cannulation. The patient is the expert of his/her own access. They should always look, feel and listen to the access7. The patients should be aware of the signs and symptoms of infection, and should never cannulate an infected AVF. They should be taught when to contact the centre for changes in the condition of the access.
- Use a tourniquet for an AVF (optional for button-hole cannulation).
- Needles with longer tubing lengths make needle removal easier. Typically the needles used for in-centre dialysis have a length of 15 cm. In order to improve ease of manipulations of needles by patient (especially for upper arm access), tubes of 30 cm are advised.
- The nurse can stabilise the access with thumb and forefinger during the first sessions.
- It is optional for a carer to stabilise the access.
- If self-cannulating, the patient has to hold the wings with thumb and forefinger and at the same time pull the skin towards him/her with the little finger.
- Taping with one tape first (to avoid accidental withdrawal of needle).
- Checking for flashback of blood/flushing.
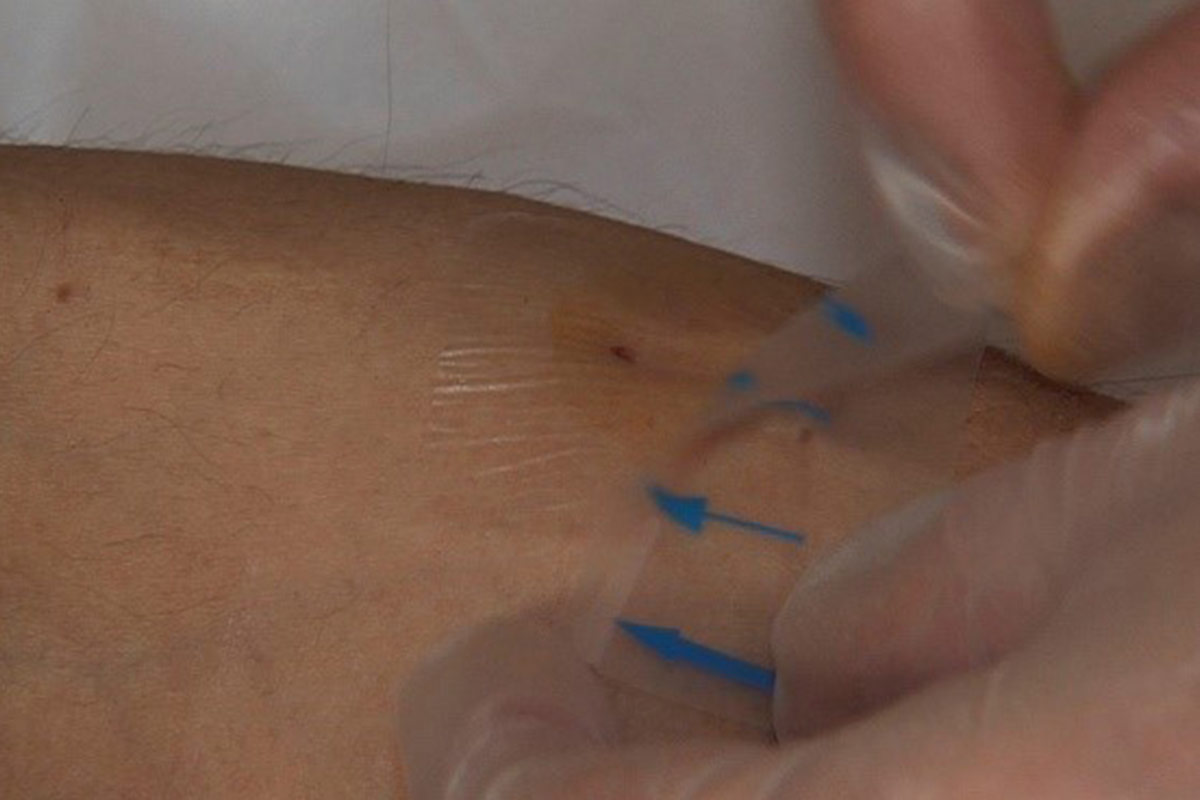
- Secure taping prevents infection and needle dislodgement. Taping at the same angle as the insertion is recommended. The Butterfly or Chevron technique is best8. Sterile tape should be used for covering the puncture site (or sterile gauze). Bloodlines should be looped loosely to allow movement of patient and to prevent bloodlines pulling on the needles.
Dialyse and disconnect
- Patients doing nocturnal dialysis or sleeping during their sessions should have a device detecting blood loss on the venous needle8 (see chapter 12).
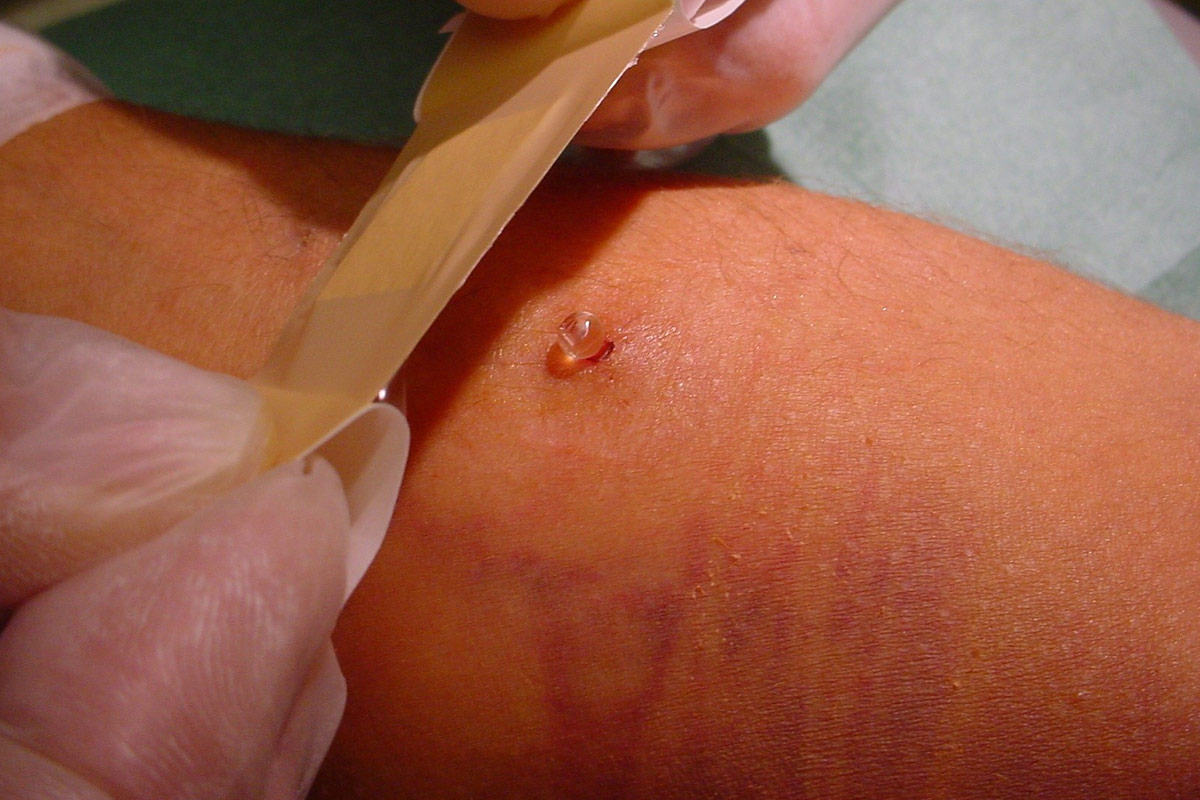
- Withdrawal of needles is an important part of the cannulation process. Upon completion of dialysis, withdrawal has to be done at the angle of insertion and mild pressure applied (once needle has been withdrawn) with one finger over the vessel insertion site10. Experience has shown that using two fingers is not always easy for the patient and that bleeding occurs if fingers are not in the right position.
- When the bleeding has stopped, the cannulations sites have to be disinfected and covered with a dressing according to unit protocol.
Evaluate
- Variations in venous and/or arterial pressure, bleeding along the needle or longer bleeding times after dialysis should be reported to the centre.
Training hints
- Discuss any concerns or worries and ensure training plan considers these.
- If training has to be started with newly developed AVF, only qualified staff should carry out first cannulations.
- If ultrasound device is available, it should be used to increase success of cannulation and determine best sites.
- It is much easier for the patient to cannulate both needles antegrade (in direction of flow) and it might be fistula protective14. Use a marker to indicate the direction of the vessel.
- Removal of the needles is often a good first step in the cannulation training process.
- Practice with an ‘artificial arm’ such as an orange.
- Patient may benefit from watching others being cannulated.
- Patient should watch himself/herself being cannulated.
- Use a ‘Tandem hand’ technique, where the hand of the patient is guided by the hand of the cannulator9.
- Practice of cannulation should be started as soon as possible during training and whenever successful cannulation seems possible.
- The length of training varies considerably, and is access and patient dependent. Prior to going home the patient has to master the procedure and feel confident.
- Encouraging and praising the patients in all phases of process is essential.
Plastic cannulae
- These are a possible alternative for metal needles in the home setting. Due to their characteristics, they seem to be well suited for restless patients, children, and patients who are allergic to metal11 and nocturnal dialysis. However, a cannulation helper is in most situations needed because it is very difficult during selfcannulation, to withdraw the inner metal needle and to manage the connections because of the absence of attached tubing.
5.2.3 Cannulation techniques (site selection)
Different cannulation techniques
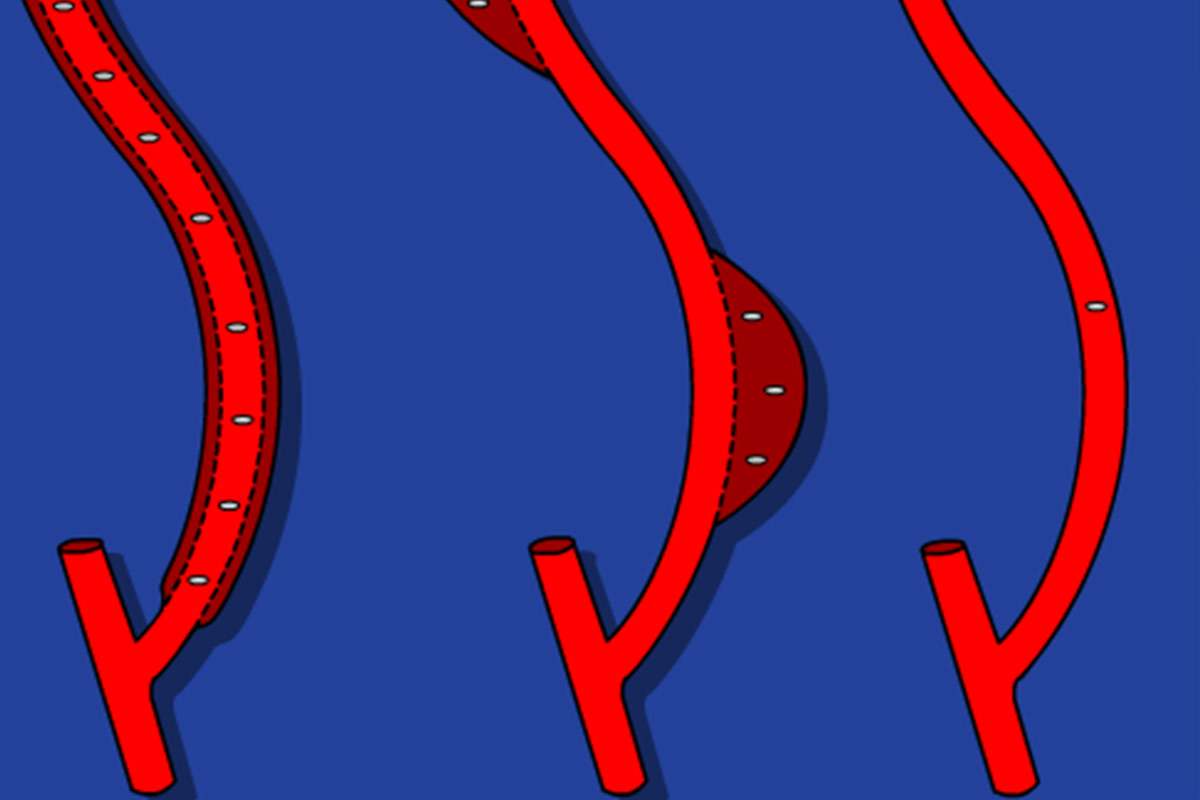
Rope ladder cannulation
The rope ladder technique is a very good cannulation technique. The whole length of the vessel is used with rotation of the puncture sites, leaving a distance of at least 5 mm between the sites. According to the literature there may be less infection risk than in buttonhole cannulation13. However, this technique has disadvantages:
- Experience has shown that patients very often don’t rotate because of fear of using different sites and length of vessel.
- It is usually done with a sharp needle which carries a risk for haematoma during connection and during nocturnal dialysis.
Area cannulation
Area cannulation has the worst outcome with thinning of the vessel wall, aneurismal formation and stenosis15,16 and should be discouraged.
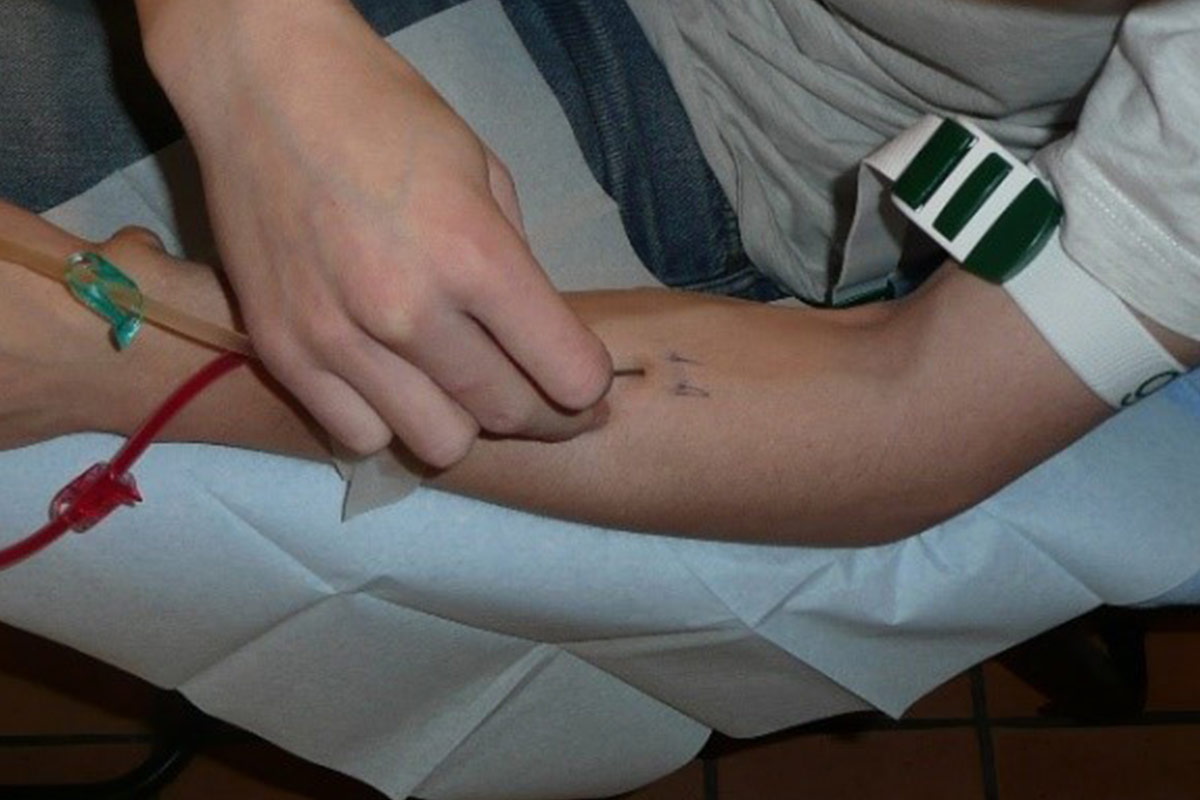
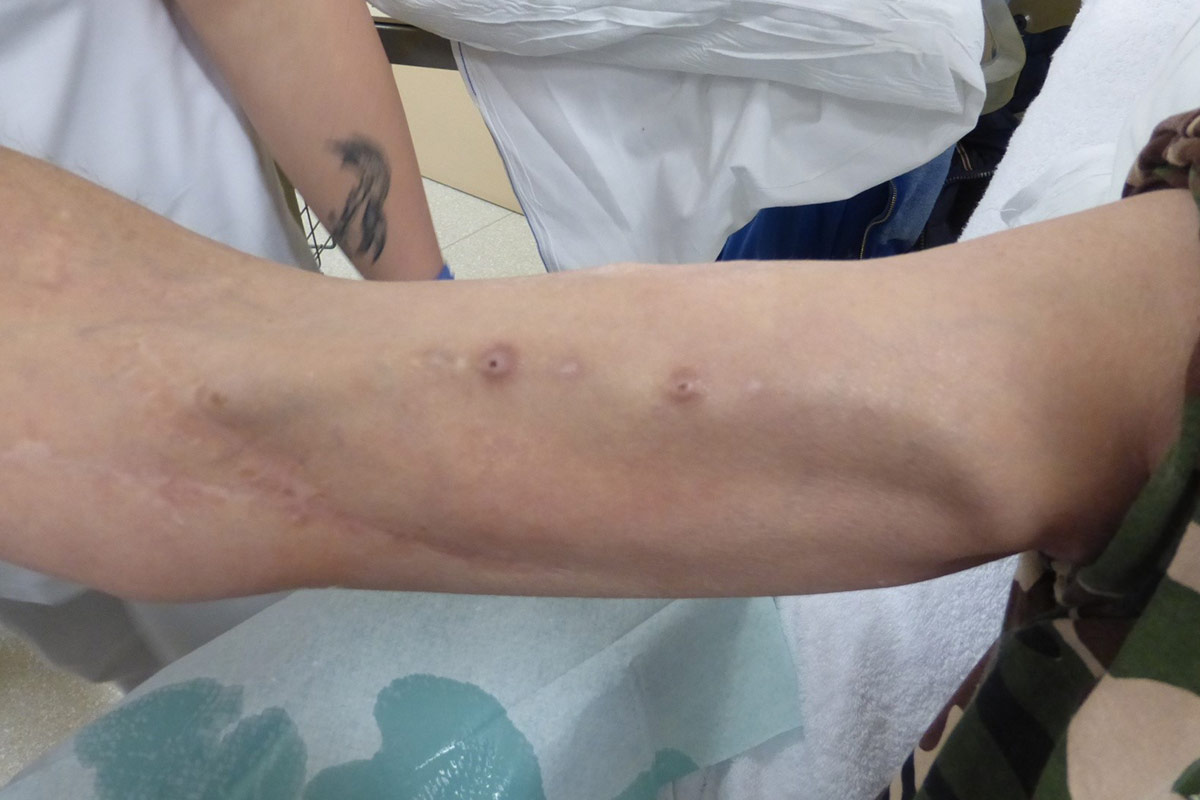
Buttonhole cannulation (BH)
The increased popularity of upper arm AVF which have short and tortuous tracks, the ageing population on HD, the high prevalence of vascular co-morbidities and the rise of frequent HHD have all increased the interest in the BH17. The BH technique is a cannulation procedure where the AVF is cannulated in the exact same spot, at the same angle and depth of penetration at each dialysis session.
There are many papers reporting an increased infection rate18,19,20. However, many variations in cannulation technique existed among studies, and many descriptions of the procedure were incomplete and unclear. In addition, since the publication of some of the papers, the technique has been evolving. However, to prevent infections it is mandatory to strictly adhere to a clear protocol.
The British Renal Society Vascular Access Special Interest Group recommends screening and selection of patients to undergo buttonhole cannulation:
- All patients undergoing buttonhole cannulation should be screened for MRSA and MSSA including their arteriovenous fistulae site, a minimum of every 3 months.
- Decolonisation can be done for those with MSSA (Methicillin-Sensitive Staphylococcus Aureus).
- Patients should be individually risk assessed by the renal team before initiating buttonhole cannulation21.
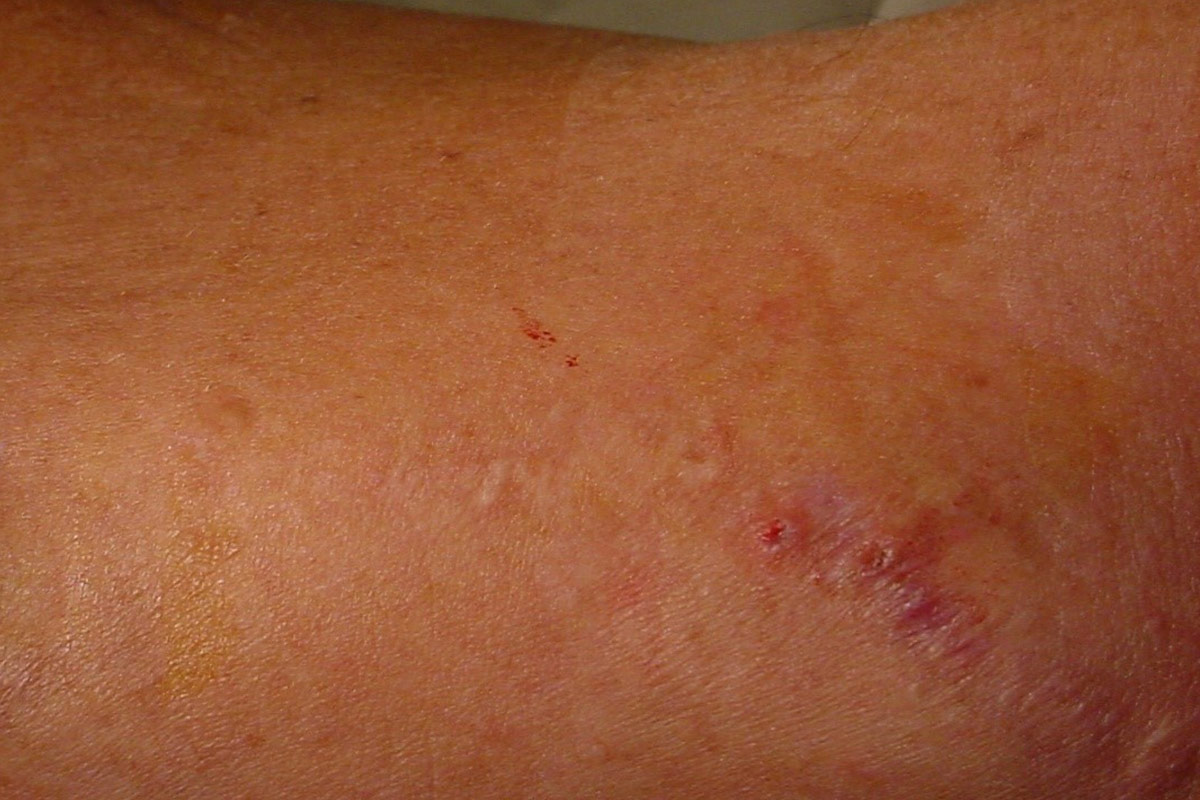
Special attention must be given to patients with a previous access infection since they may be prone to infection recurrence22.
Cannulation variation
Most aspects of cannulation are identical to standard cannulation but consider these extra aspects:
- Involving the patient during the creation of the tunnel track is not easy, usually training for self-cannulation is started subsequently, when blunt needles can be started.
- If ultrasound device is available, it should be used to increase success and to search for ideal, healthy sites to create buttonholes.
- The sites should be easy to reach by the patient and a distance of at least 4-8 cm in between is recommended.
- It is strongly advised that the procedure is performed by the same cannulator/trainer, until the tunnel track has been created. It takes at least 6 cannulations with a sharp needle before dull needles can be used.
- Some HHD centres create two arterial and two venous puncture sites in case of extensive dialysis.
- Washing/scrubbing of the arm with surgical or exfoliating sponge makes scab removal easier (in some cases scabs are completely being removed during process!) and would have a favourable effect on the infection rate23.
- Do first disinfection (attention contact time), then complete scab removal with a blunt needle (not cannulation needle) or scab remover (comes with blunt needle) then second disinfection (attention contact time).
- During cannulation it is mandatory to keep the arm in the same position and on the same angle in order to align the needle with the tunnel track. It therefore can be helpful to train the BH cannulation with the arm on a table. The patient can continue the same procedure at home.
- Touch cannulation Instead of holding the needle by the wings, it is held by the tubing to prevent cannulator using force and to push the needle in a wrong direction. The needle will follow more easily the direction of the tunnel track, without damaging it. The so called ‘trampoline effect’, where the needle point bounces against the vessel wall, will be less frequent.
- If resistance is met or the needle bounces back, the patient has to be instructed to gently withdraw the needle in the tunnel until the bevel reaches its entrance and try again (recheck the position of the arm). If the needle has been removed completely outside the tunnel, it should be replaced by a new one.
- If the cannulation is unsuccessful after two attempts (with different needles), the patient should have a rest and retry 15 minutes later. An alternative is to postpone, if possible, the dialysis till the next day6. It is generally recommended not to use a sharp needle if cannulation with a blunt needle is unsuccessful however, it can be allowed if the patient inserts a sharp very gently, using ‘touch cannulation’ through the tunnel track. Careful use of a sharp needle does not increase complications24.
- Hubbing is when the hub of the needle is pushed into the cannulation site causing the needle to stretch the tunnel entrance. The scab becomes difficult to remove, increasing the infection risk. Therefore, 1 to 2 mm of the metal of the needle should be visible after cannulation25.
- If a tourniquet is used, caution has to be paid not to tear the skin and change the direction of the tunnel track!
- In patients with deep or unstable vessels a subcutaneous vascular needle guide ’VWING’ can be surgically implanted on the vessel wall. It guides the needle directly to the vessel through the same pathway every time, rapidly enabling the use of blunt needles and facilitating self-cannulation in these patients26.
Developing the buttonhol
An easier way to obtain a straighter tunnel track is the use of a plastic peg. As long as pegs are used, there is no scab formation. When the needle after dialysis has been removed, and bleeding has stopped the puncture site is disinfected and the peg is inserted. It remains in place until the next HD session covered with a compressive dressing. When the peg is removed just before the next session, a sharp needle is gently inserted along the track already formed by the peg. These steps are repeated at least 6 times. The length of the peg can be adapted to the depth of the vessel wall.
Using the pegs, it is not mandatory to have the same cannulator during the whole process. This way the patient can in some cases start self-cannulation earlier. It takes less courage to slide the needle through an open tunnel track (Ideal for patients with needle fear).
Post dialysis care
- When the bleeding has stopped, the cannulations sites have to be disinfected and covered with a dressing. In order to control infections, some centres apply topical 2% Mupirocin ointment to the punctures sites27.
- Two procedures decrease formation of scab and might be infection protective:
- Moist healing method28 leads to formation of no scab or a very small scab. When the bleeding is stopped, the site is disinfected with a diluted povidine iodine solution and covered with a sterile plastic dressing for 24h to keep it moist. During the scrubbing procedure, before the next dialysis session, the scab, if any, will be in most cases easily removed.
- Silver coated dressing has an antibacterial action and decreases the size of the scab.
5.3 Arteriovenous graft (AVG)
- AVG is a good option if creation of an AVF is not possible, although there is an increased risk of complications.
- The ideal place for an AVG in a HHD patient who self-cannulates, is the non-dominant forearm. Upper arm AVG and certainly AVG on the dominant arm, are more difficult to self-cannulate.
- The recommended cannulation technique for AVG is the rope ladder technique and there is no evidence supporting the use of BH29. However, some centres apply the buttonhole technique in grafts with good outcomes (based on the experience of theauthor).
Cannulation variation
- A tourniquet is not required.
- All other recommendations for AVF rope ladder cannulation are appropriate for AVG.
- For more general information on AVG, see EDTNA/ERCA publication on ‘Vascular Access Cannulation and Care – A Nursing Best Practice Guide for Arteriovenous Graft’29.
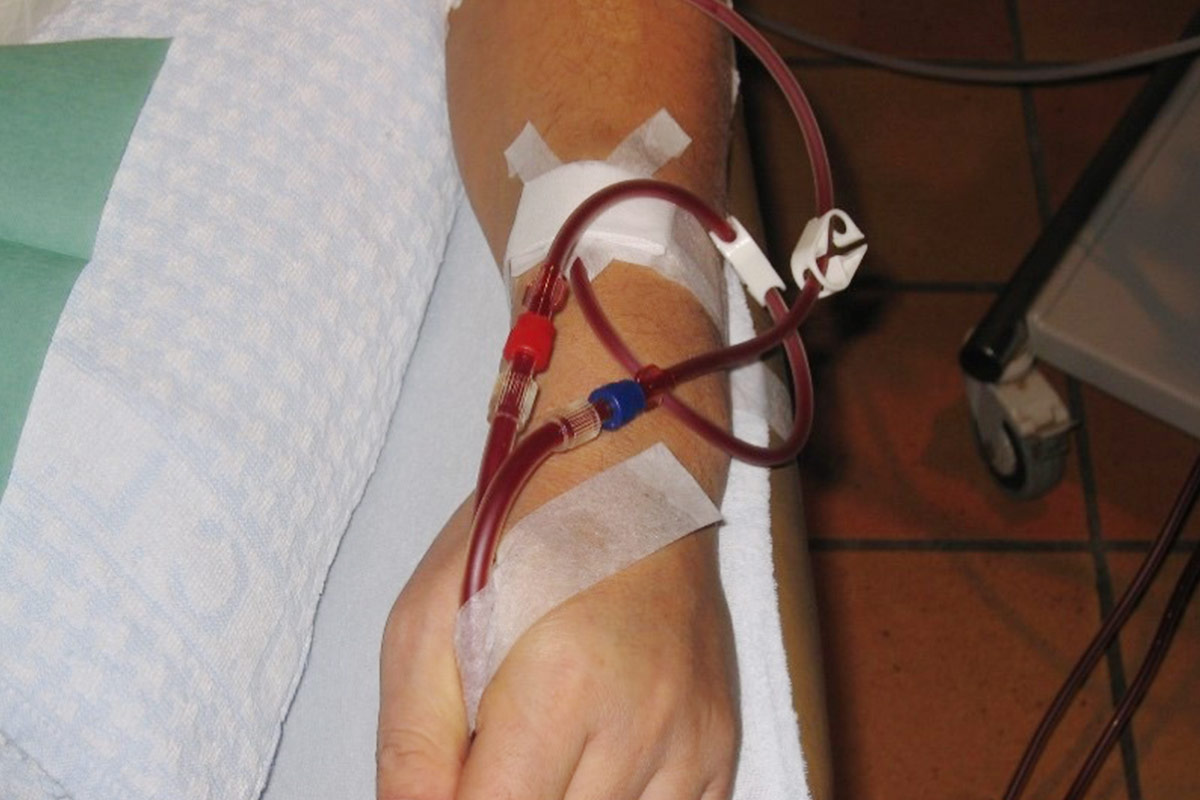
5.4 Central Venous Catheter (CVC)
There are many different catheters available. They should be able to provide a minimum blood flow rate of 300 ml/min consistently in order to maintain adequacy of dialysis10. However, there is no evidence to guide selection of one type of CVC over another.
- CVC use in patients on HHD is, as well as for patients undergoing in-centre dialysis, associated with higher risk for mortality and hospitalisation30,31,12. Canadian guidelines for the patients treated with intensive HD suggest the use of AVF or AVG over CVC32.
However, studies reported good outcomes with use of CVC in intensive HD33,34,35,36,37,38. Also in the KIHDNEy European cohort 24% of patients were at home with a CVC49. - In some countries, the use of CVC is not allowed or controversial in the home setting.
- CVC allows a more secure connection to the extra-corporal circuit. It is therefore the preferred access for Nocturnal HD in some HHD programmes39.
5.4.1 Practical instructions for successful CVC handling
- Adherence to strict aseptic technique is mandatory when handling CVC and procedures should be consistent with current evidence base10:
- Proper hand washing (with liquid soap) is mandatory before starting.
- Disinfect hands with an alcohol based rub.
- Wearing mask is advised (patient and carer).
- Whatever disinfectant agent is used, the contact time has always to be respected.
- Avoid traction on CVC. During treatment lines have to be secured to prevent trauma.
- Patients doing nocturnal dialysis or sleeping during their sessions may have a device detecting blood loss on the venous hub (see Chapter 12 on risk).
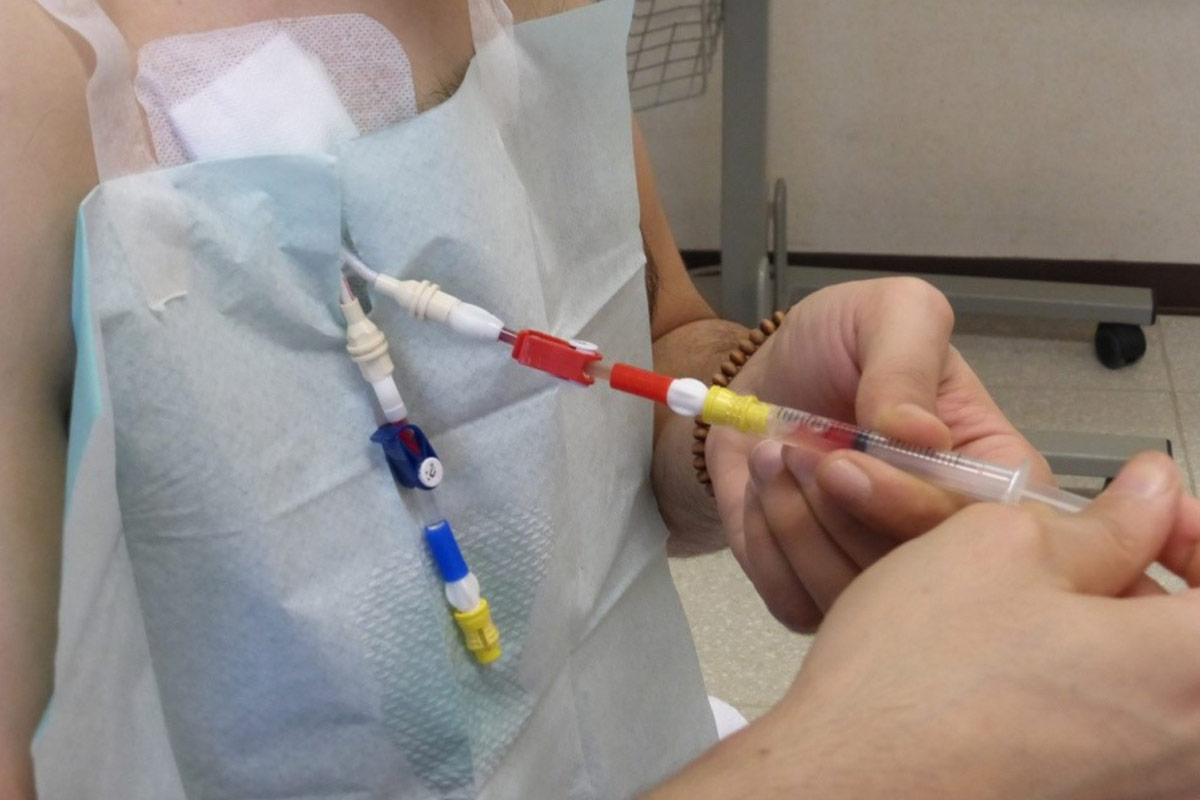
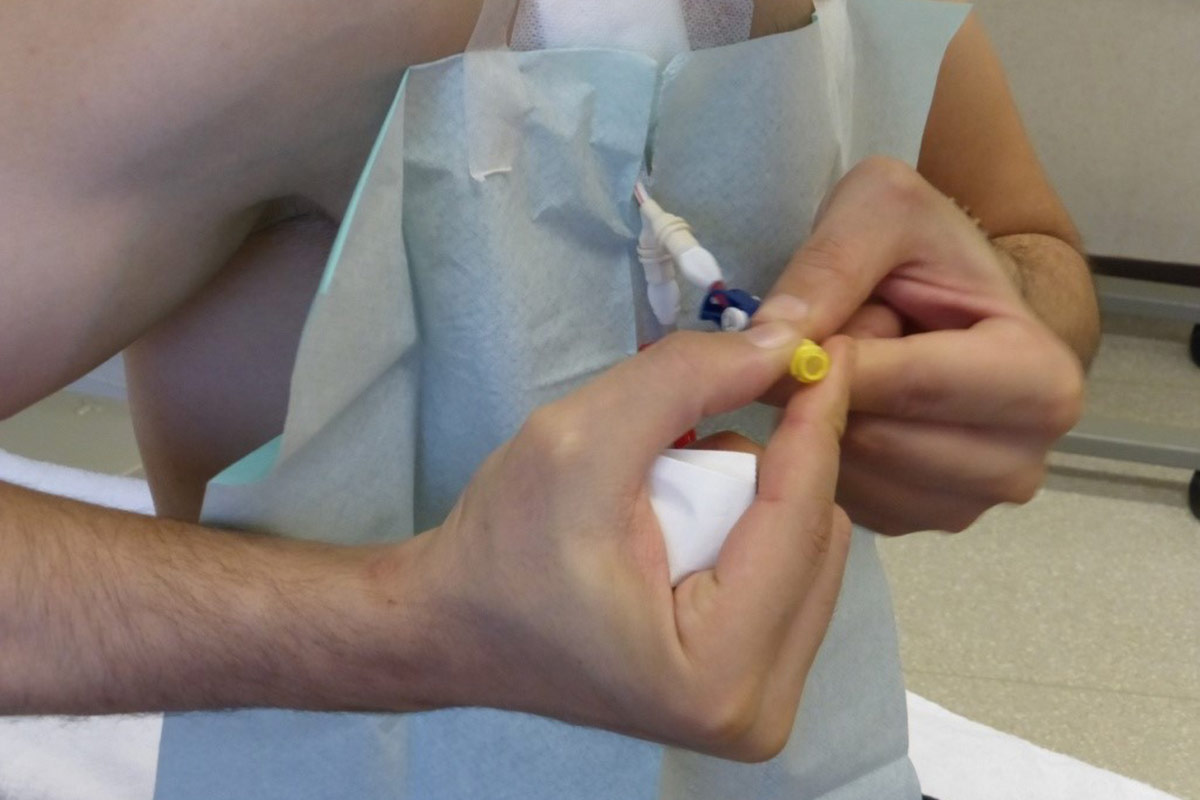
5.4.2 “Closed connector” or “closed luer access” devices
Various connectors are currently marketed which create a mechanical and microbiological closed system implicated in decreasing blood stream infections for CVC40,21. The Canadian Society of Nephrology guidelines for Intensive HD, recommend a closed luer device for patients receiving intensive HD18. However, it is recommended to use the device in all HHD patients with CVC41,42. It protects against accidental disconnection, avoiding bleeding, and last but not least air embolism (negative pressure when patient is not in supine position). In some cases, flushing with 0,9% saline after dialysis is sufficient to keep the CVC patent and avoid injection of an additional lock solution.
However, if not connected properly, the device can also lead to accidental disconnection and related complications, including air embolism and bleeding.
- The devices have to be changed according to unit protocol (aseptic technique).
- At each dialysis session, the patient has to check if connections between the hubs of the CVC and the device are still tight.
- Despite the device, the lines of the CVC always have to be clamped.
5.4.3 Locking of CVC
Traditionally each lumen is flushed with 10–30ml of saline after dialysis prior to locking with heparin/anticoagulation/antimicrobial solution. Each unit has their own protocol. Expert opinion is advocating for increase in volume of flushes (to 30ml) to minimise formation of secondary fibrin membrane and flow issues43. In order to avoid aspiration of blood in CVC, it is mandatory to clamp lumen at each time before removing syringe and leave it closed till next dialysis session44.
At each dialysis session the lock solution has to be removed completely and then flushed with 0,9% saline. The centre should be contacted if the lock cannot be removed.
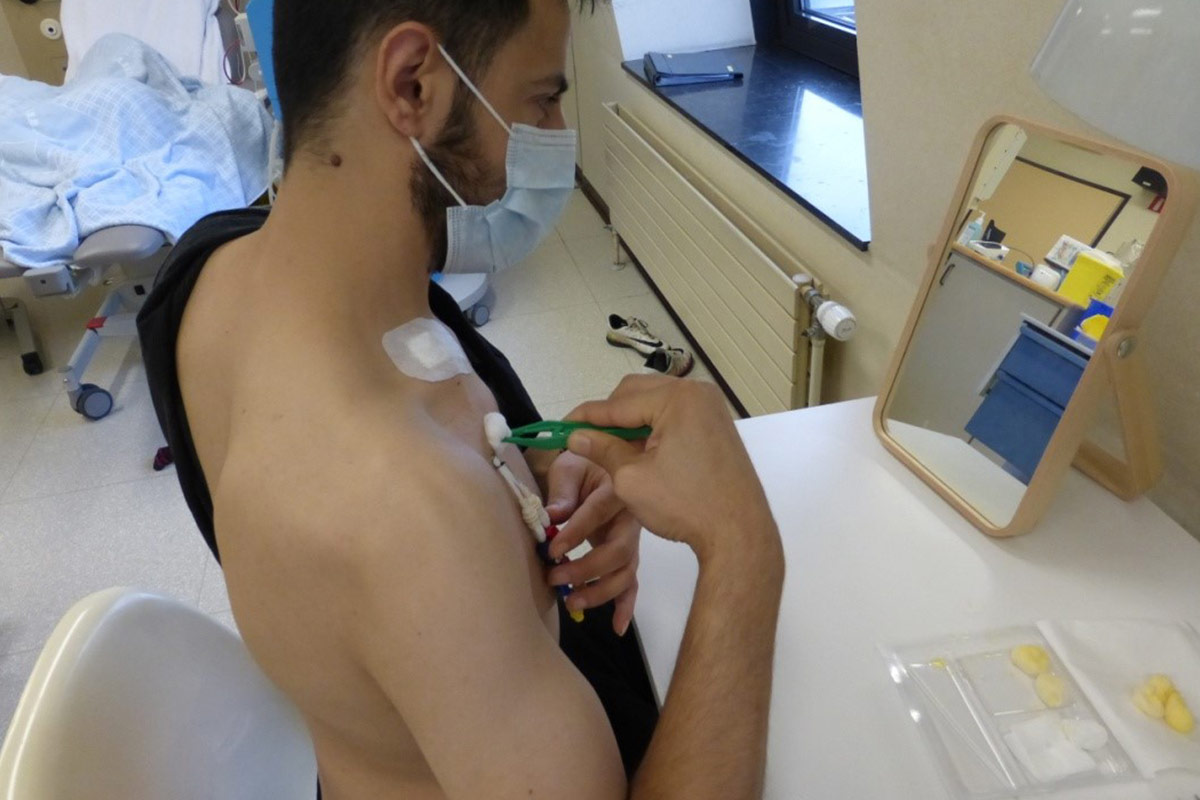
5.4.4 Dressing
- Many types of dressing are available for the exit sites of CVC. They protect against infection and eventual trauma and can, if waterproof, be left on during shower.
- There are different protocols for showering with CVC, with or without protective dressing. The water source can be a source of infection. Also shower and shower head are potential sources of bacteria; therefore, regular cleaning of both is recommended46.
- Absence of dressing can be an option for patients with allergic reaction to all kinds of tape (extreme caution) but the catheter should be secured. This procedure is used in some PD programmes45.
- Inspecting for signs of infection at each dressing is mandatory43. The HHD unit must be contacted if signs of infection are present.
- Frequency of dressing changes is according to unit protocol, ranging from each session or after shower, to weekly.
- Strictly adhere to prescribed protocol (disinfectant, type of dressing).
- Teaching of dressing change can be done in front of mirror for patients who want to do it themselves.
5.4.5 Complications of CVC
Unfortunately, CVC flow dysfunction occurs regularly and leads to decreased dialysis efficiency. Definitions of catheter dysfunction vary, but in general they relate to the inability to achieve a certain blood pump speed within the venous and arterial pressure limits of 250 and –250 mmHg, respectively, while dialysing46. A blood flow change of more than 20% over three consecutive treatments is an indication of a problem47, or if inversion of dialysis lines due to flow problems is regular practice16. Inversion of lines however increases recirculation so is not recommended.
- The patency of the catheter must be checked during training as reference point for follow-up in the home setting.
- The centre has to be contacted if dysfunction, as defined above, is detected.
- Flushing of lumens with 10 to 30 ml of 0,9% saline (push and pull movement) can be effective to clear poor flows.
- Administration of a thrombolytic may be necessary. Some centres allow the patients to administer a thrombolytic at home, others request the patient to come to the unit.
CVC infections occur more frequently than infections of arteriovenous access. They can be local (exit site or tunnel infection) or systemic. Patients must be taught the signs of infection and should check their temperature each treatment or daily. If signs of infection are observed, the patient has to come to the centre for swabbing of pus and or sampling of blood. Topical or systemic treatment has to be started according to centre protocol.
Summary
All types of vascular access can be used in the home setting. A wellfunctioning vascular access is a key success factor for HHD. Care of the access is a very important part of the training programme.
Adherence to the unit protocol is extremely important.
Retraining, observation of the technique during home visits and motivation of the patient, are helpful for infection control and overall survival of the access.
Learning Activity
- Which vascular access types can be used in the home setting?
- Give a short description of the different cannulation techniques with advantages and disadvantages.
- What are the advantages of self-cannulation?
- What is a “closed connector device”?
EDTNA/ERCA Secretariat
E-mail: secretariat@edtnaerca.org